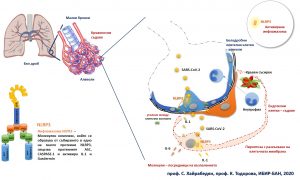
Studies launched in 2010 by scientists at the Institute of Biology and Immunology of Reproduction at the Bulgarian Academy of Sciences (IBIR-BAS) suggest that it is the overreaction of a protein complex called the NLRP3 inflammasome that underlies the severe complications with COVID-19. The inflammasome is a molecular sensor in cells that responds to a meeting with a pathogen and / or cellular damage resulting in the release of inflammatory signals, and a particular type of cell death, pyroptosis, can occur. In doing so, the cells burst and release a huge amount of inflammation and danger signals to which neighboring tissue cells and cells of the immune system respond. Normally, dead cells are eaten by cells of the immune system such as macrophages but in this case, the signals travel much further.
The inflammasome is part of the mechanisms of the innate immune response and for many years was thought to be present only in immune cells. It has recently become apparent that such a complex is present in other cells, too, such as those in the respiratory tract as well as the cells that build the blood vessels. Prof. Dr. Soren Hayrabedyan and Prof. Krassimira Todorova of IBIR-BAS showed for the first time in science literature in 2016 (Nature Sci Reports 2016) вthe possibility for this inflammasome to get activated even in epithelial cells – an immunologically tolerant environment which signalled inflammation and cell death. Two years later, this data was also confirmed in patients by a team led by Ludwig Maximilian University at Max Planck (Reproduction, 2018).
The first evidence of severe respiratory failure resulting from the so-called “cytokine storm” and sepsis led us to believe that namely this molecular complex may be related to it since blocking the inflammasome in experimental patterns of sepsis stops the process, say the scientists. At higher viral loads or in hereditary predisposition to a stronger than normal response, the inflammasome in the epithelial cells of the lung as well as in the cells of the small vessels may be activated. It is strange that in lung cells this can lead to the release of inflammatory signals but at the same time it strengthens the contact between them. However, with adjacent micro vessels, the effect can be dramatic – significant cell death, with a burst, which can cause gas or blood flow abnormalities. All-new scientific articles from 20-23 April 2020 confirm this in patients with COVID-19, showing severe blood clotting phenomena in small vessels.
Inflammasomes are associated with various diseases in which the immune system is involved, incl. diabetes, multiple sclerosis, chronic ulcerative colitis, rheumatoid arthritis, gout and sepsis. Patterns of sepsis are interrupted by blocking the inflammasome with an inhibitor. Chloroquine, which has been tested as a potential agent against COVID-19 and given to patients with rheumatoid arthritis and lupus, is a direct blocker of the same inflammasome and may suppress a sepsis pattern. Most often cited are its mechanisms of action established in the 80-90 years of the last century, since inflammasomes were only discovered recently.
The hypothesis of the two scientists of IBIR could explain both the cases in young people – the overactivation of inflammasomes as well as the more severe course of the disease in people with high blood pressure, diabetes, atherosclerosis since in the latter, it is precisely the cells of the small vessels that are affected first. It is important to note that inflammasomes are involved in the very pathogenesis of diabetes and atherosclerosis and are already active. The blockage of inflammasomes leads to the stoppage of vessel inflammation patterns in Kawasaki disease, which causes coronary vessels inflammation and fever.